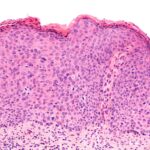
There is always a risk of an HPV-infected person suffering from a persistent (or chronic) viral infection. A persistent HPV infection usually causes long-term symptoms and health consequences, including the development of lesions and cervical cancer. The worst part is that few treatments exist to combat HPV-persistent infections.
A recent Italian research study tested the effects of a new treatment on patients suffering from persistent HPV infections and lesions on their cervixes. The treatment was called Pervistop®, which contains a combination of EGCG (epigallocatechin gallate), Vitamin B12, hyaluronic acid, and folic acid.
For consumers in the US who want a comparable treatment they can take HPD Rx ONE which is an HPV supplement which also contains EGCG, B12, and Folate as well as 28 additional vitamins, minerals, and phytonutrients.
The study involves forty adult female participants suffering from HPV-persistent infections and cervical lesions. The participants were divided into two groups, one receiving the Pervistop® treatment and the other receiving no treatment. The treated group was given approximately 400 mcg of folic acid, 200 mg of EGCG, 50 mg of hyaluronic acid, and 1 mg of Vitamin B12 for around three months (12 weeks). The control group was the group that did not receive any treatment.
After 12 weeks, an LSIL (low-grade squamous intraepithelial lesion) still existed in 15 out of 20 participants in the control group (the untreated group). As for the treated group, approximately 17 out of 20 participants showed no signs of cervical lesions or the virus in their system. Signs of the HPV infection apparently had been cleared out within those 12 weeks.
This data is very promising for other people with HPV-persistent infections. The combination of EGCG, Vitamin B12, hyaluronic acid, and folic acid seems to be very effective in stopping the persistence and growth of an HPV infection in the body. However, more randomized placebo-controlled studies need to be performed to see if there is any consistency in these results on other patients.
About the Study
The Italian HPV and Pervistop® study was the first controlled clinical trial using the experimental Pervistop® treatment. The Clinica ALMA RES recruited 40 female participants for the study, which was conducted between June 2022 and August 2022. It was a controlled study upheld by the highest ethical standards to ensure accuracy.
Future structured studies still need to be conducted to verify the promising results of the initial controlled clinical trial. If Pervistop® can help treat more female patients and prevent them from developing severe cervical cancer or lesions, then it could be a treatment that will help many HPV-infected women worldwide.