Cervical Cancer Screening Overview
The global market share of cervical cancer screening is currently dominated by North America, with a forecasted compound annual growth rate (CAGR) of 12.6% throughout the following years.
Valued at roughly US$ 1.4 billion in 2022, the global market for cervical cancer screening is predicted to experience a CAGR growth of approximately 13.7% over the projected period. By 2033, the value will reach a whopping US$ 5.7 billion.
The surge in new cervical cancer patients and existing patients exhibiting the disease has been remarkable. According to the American Society of Clinical Oncology, the rise in new patients across five cancers has been significant compared to pre-pandemic numbers. More specifically, cervical cancer recorded a 20% increase. However, cervical cancer can be prevented with vaccines, early screening tests, and prompt therapeutic intervention of the human papillomavirus (HPV).
Some countries with limited medical access, such as parts of Africa, highlight the importance of universal healthcare access. Companies are proactive in coming up with innovative technological solutions to empower patients and increase their involvement in their health management. One such advancement is the mobile health application iThemba Life, which offers users HPV viral load information, educational resources, reminders, and support directly on their smartphones. This initiative provides patients with healthcare access and enhances engagement for individuals in resource-challenged areas.
These developments accelerate the growth of the cervical cancer screening market, projecting to maintain the demand for advanced and innovative treatment solutions in the foreseeable future.
Key Market Insights
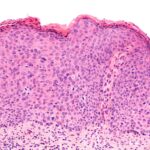
HPV is the dominant segment of tests among end-users, securing approximately 75.0% market share in 2022. For risk control, HPV testing offers significant insights. There are various forms of HPV, including high-risk HPV and low-risk types. While low-risk HPV types generally precipitate benign genital warts, high-risk HPV strains correlate with an increased risk of cervical cancer.
Regarding cancer types, squamous cell carcinomas are the most common and are strongly linked to high-risk HPV infections (HPV strains 16 and 18).
The age bracket of 31 to 65 years accounted for the maximum market share of 61.7%. This age range marks a period when the cumulative exposure rate to HPV has increased significantly.
The hospital segment, in terms of end-users, constituted a revenue share of 39.7% in the global market as of the end of 2022. Hospitals frequently possess advanced diagnostic amenities, encompassing imaging techniques, laboratory tests, and access to specialized tests. These resources can facilitate a comprehensive diagnostic workup and aid in accurate diagnosis formation.
North America continues to lead the market of cervical cancer screening, with an expected CAGR of 12.6% during the anticipated years. Key drivers of the cervical cancer screening market include the rising implementation of screening for early identification of cervical cancer, progresses in detection, and management for improved prognoses.
Competition in the Market
Key players in the cervical cancer screening industry aim for market expansion through growth strategies (e.g., acquisitions, mergers, partnerships). They focus on boosting sales and leveraging novel technologies to innovate product development.
In October 2022, QIAGEN announced the certification of their leading in-vitro diagnostic (IVD) kit and NeuMoDx platforms, in compliance with the In-Vitro Diagnostic Medical Devices Regulation (IVDR) in Germany.
Everlywell, an Austin-based company, significantly expanded its reach in March 2021 with double acquisitions and restructuring that led to the creation of a parent company, directed by Everlywell CEO and co-founder Julia Cheek.
Risk Factors for HPV
Certain people have a higher risk of contracting HPV. These include:
- Individuals who are sexually active with a new partner
- Those with multiple sex partners
- Individuals engaging in sexual activities with someone who has or had multiple partners
- Those with a compromised immune system, such as individuals with human immunodeficiency virus (HIV)
HPV Vaccine
Ever since the release of the HPV vaccine, the rate of HPV cases gradually decreased. The Centers for Disease Control and Prevention (CDC) claim that full vaccination can prevent over 90% of cancers triggered by HPV.
Gardasil-9 is currently the sole HPV vaccine available in the United States. It shields against two low-risk HPV types (6 and 11) and seven high-risk types (16, 18, 31, 33, 45, 52, and 58). Although Gardasil-9 cannot treat existing HPV infections, it can protect against future infections from different HPV strains.