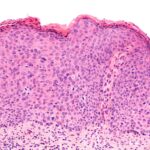
The Papanicolaou (Pap) smear is the standard test for cervical cancer screening in the United States and worldwide. The U.S. began Pap test screening in the 1950s, and within thirty years the rate of cervical cancer decreased by 70 percent.
Doctors perform Pap smears (also called Pap tests or cervical cytology) during routine cervical examinations. An instrument called a speculum is inserted into the vagina, holding the walls apart to allow the doctor to inspect the cervix. Using a soft brush and a small scraping device called a spatula, the doctor obtains a sample of cells from the cervix and transfers the cells into a container holding a liquid preservative or onto a glass slide. The sample is then sent to the laboratory where a pathologist examines the cells under a microscope and determines whether they have abnormal features placing the patient at risk for cancer. Two different cell types are examined: squamous cells and glandular cells.
What Does A Pap Smear Test?
A Pap smear report defines the squamous and glandular cells based on their features. These features distinguish whether the cells have likely been infected by human papillomavirus (HPV), the virus that causes cervical cancer. Depending on these results and other factors, including the patient’s age and history, follow-up testing may be necessary. A Pap smear report can have the following outcomes:
- Negative for intraepithelial malignancy (NILM): The cells are normal.
- Atypical squamous cells of undetermined significance (ASC-US): The cells have some abnormalities, but they are not severe enough to be classified as lesions caused by HPV. This is the most common abnormal finding on a Pap smear.
- Low–grade squamous intraepithelial lesions (LSIL): The cells demonstrate mild abnormalities caused by low-risk HPV types (usually HPV 6 and 11). For many (but not all) women, this is a temporary finding and becomes normal within 1-2 years.
- High-grade squamous intraepithelial lesions (HSIL): The cells demonstrate moderate or severe abnormalities caused by high-risk HPV types (usually HPV 16 and 18). These cells are more likely to indicate precancer and to progress to cancer.
- Atypical squamous cells cannot exclude high-grade squamous intraepithelial lesions (ASC-H): The cells have abnormalities that may or may not indicate HSIL, and further testing is needed.
- Atypical glandular cells (AGC): The glandular cells (cells responsible for producing mucous) are abnormal and may suggest either inflammation which can resolve on its own, or potentially precancer or cancer of either the cervix or the uterus.
- Adenocarcinoma in situ (AIS): The glandular cells are precancerous and may become a cancer called adenocarcinoma if not treated.
- Cervical cancer: This is called squamous cell carcinoma if the squamous cells are affected or adenocarcinoma if the glandular cells are affected. Cancer is a very rare finding for women who have regular Pap smears because less severe cellular changes are usually caught and treated before cancer develops.
Depending on the patient’s risk factors, the most common follow-up test if a Pap smear is abnormal is called a colposcopy. During this procedure, the doctor uses a speculum to open the vagina and applies a vinegar solution to the cervix; if certain spots appear white, this indicates that there are abnormal cells in those areas. The doctor then takes samples of those cervical areas (called a cervical biopsy) and sends them to the laboratory for further examination by a pathologist.
Like Pap smear results, biopsy results can also vary. Abnormal cells on a cervical biopsy are called cervical intraepithelial neoplasia (CIN) and graded on a scale of 1 to 3 (from least to most severe). Hence the different outcomes of a biopsy include CIN1, CIN2, CIN3, or cancer. Usually, but not always, Pap smears that show ASC-US or LSIL correspond to biopsies that show CIN1 (which usually resolves without treatment), and Pap smears that show HSIL correspond to CIN2 or CIN3. CIN2 can sometimes resolve without treatment but may require more frequent monitoring because they can progress to CIN3, which carries a high risk of cancer and requires treatment.
Historically, Pap smear results were used alone to determine whether follow-up testing was needed. However, in 2019, the American Society of Colposcopy and Cervical Pathology (ASCCP) introduced new guidelines that account for the patient’s risk factors along with their Pap smear results to decide what follow-up steps are needed.
Additional testing and treatment are recommended depending on the patient’s risk of having CIN3. This risk, in turn, depends on both the patient’s Pap smear results and other information such as the patient’s HPV test results and history of Pap tests. For example, if the patient’s risk of having CIN3 is under four percent, then surveillance screening is recommended. If the patient’s risk is over four percent, then either colposcopy or treatment is recommended depending on the exact risk percentage.
Since many different combinations of patient outcomes and follow-up steps are possible, the ASCCP has developed online and app-based tools for both doctors and patients to determine a patient’s risk of developing CIN3 as well as recommended action steps.
Patients who do not require colposcopy or treatment can undergo either routine screening or surveillance screening using Pap smears, HPV tests, or a combination of the two. Surveillance screening is done at more frequent intervals compared to routine screening and applies to patients who have more than average risk of developing cervical cancer. Routine screening is done at standard intervals and applies to patients deemed to be average risk.
The best way to prevent cervical cancer is to receive regular Pap smears and HPV tests at the appropriate intervals based on national guidelines. Depending on what these tests show over time, the correct steps can then be taken based on one’s risk of developing CIN3.
Sources:
Crum, C.P., Huh, W.K., & Einstein, M.H. Cervical cancer screening: The cytology and human papillomavirus report. In: UpToDate, Post, TW (Ed), UpToDate, Waltham, MA, 2023. (Accessed on October 1, 2023).
Goodman, A., Huh, W.K., & Einstein, M.H. Cervical cancer screening: Risk assessment, evaluation, and management after screening. In: UpToDate, Post, TW (Ed), UpToDate, Waltham, MA, 2023. (Accessed on October 1, 2023).
Mayo Clinic Staff. (2022, June 18). Pap smear. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/pap-smear/about/pac-20394841.
National Cancer Institute. (2023, May 5). HPV and Pap Test Results: Next Steps after an Abnormal Cervical Cancer Screening Test. https://www.cancer.gov/types/cervical/screening/abnormal-hpv-pap-test-results